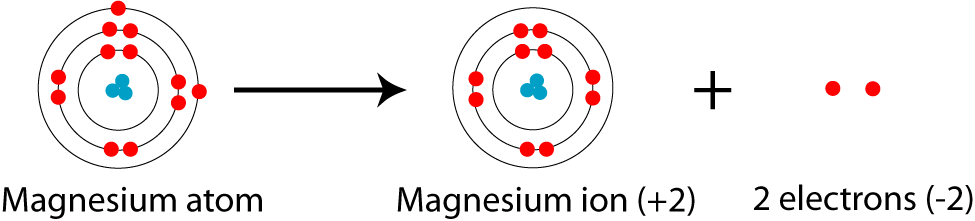
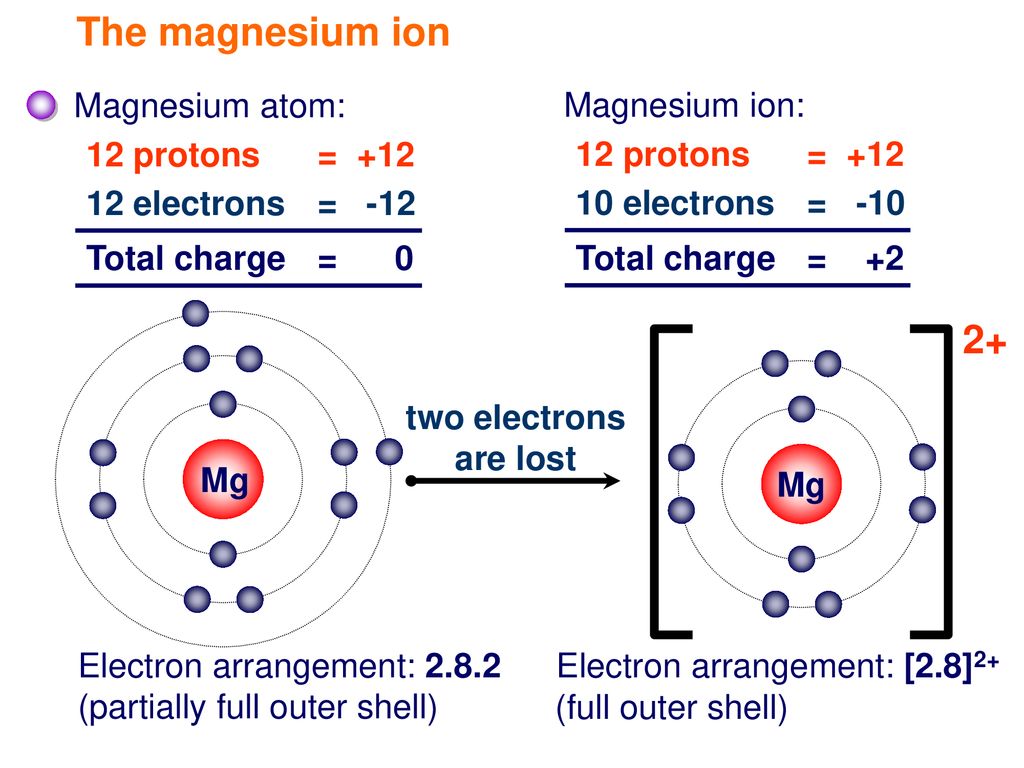
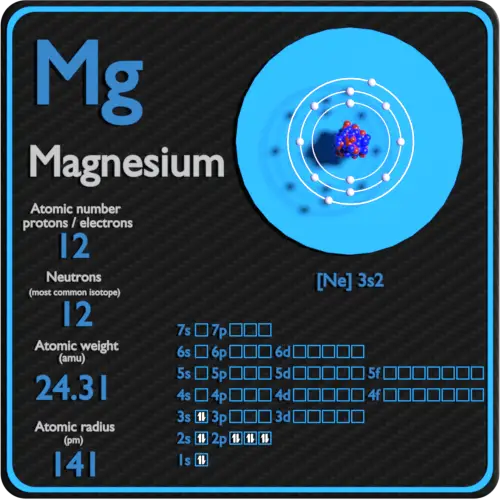
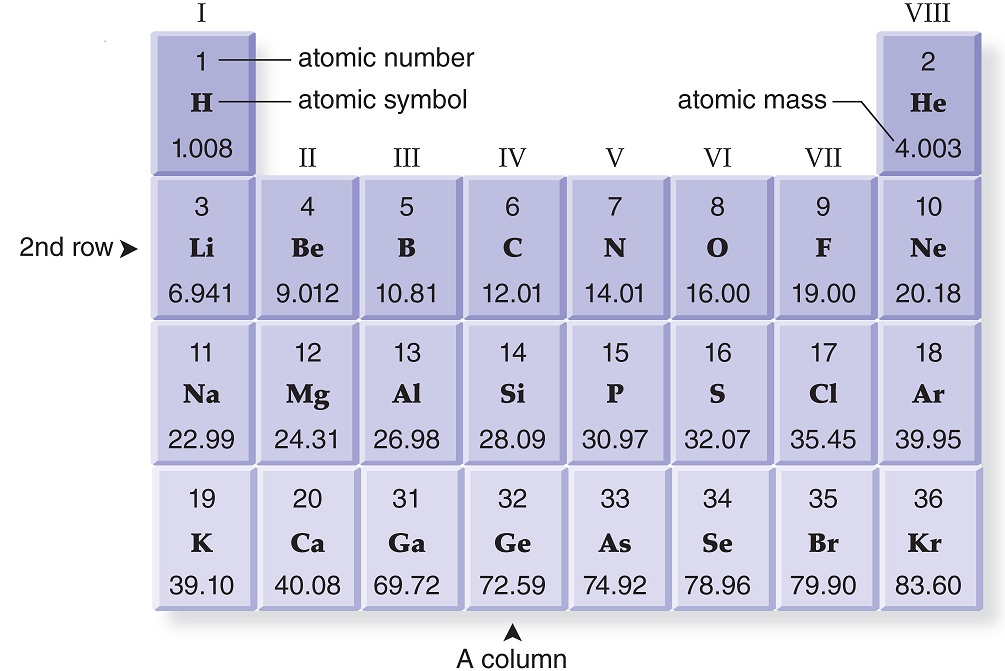
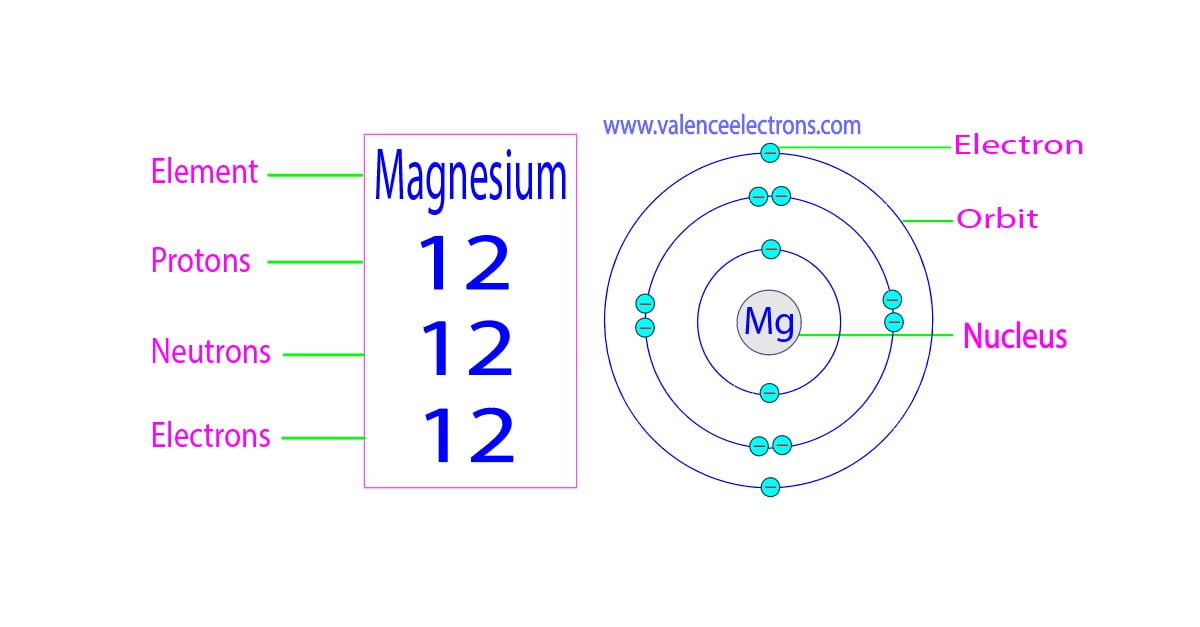
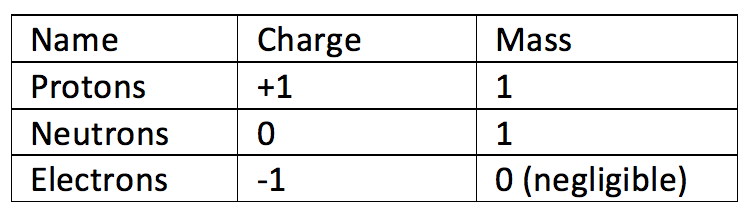
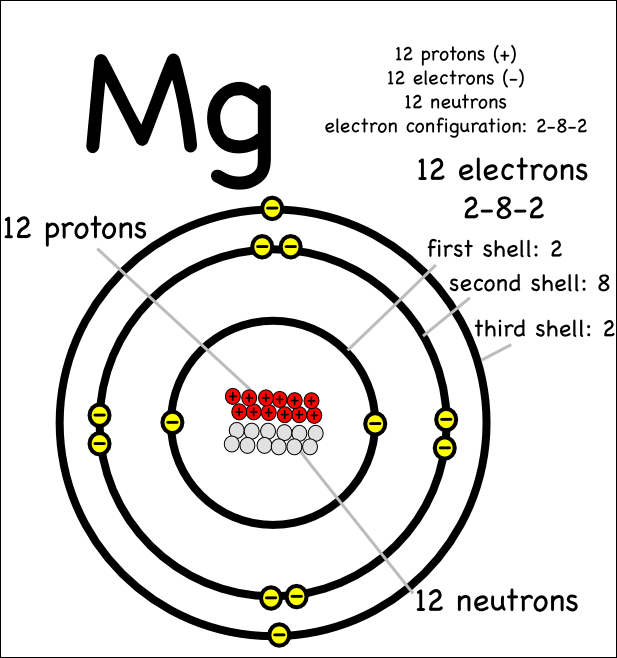
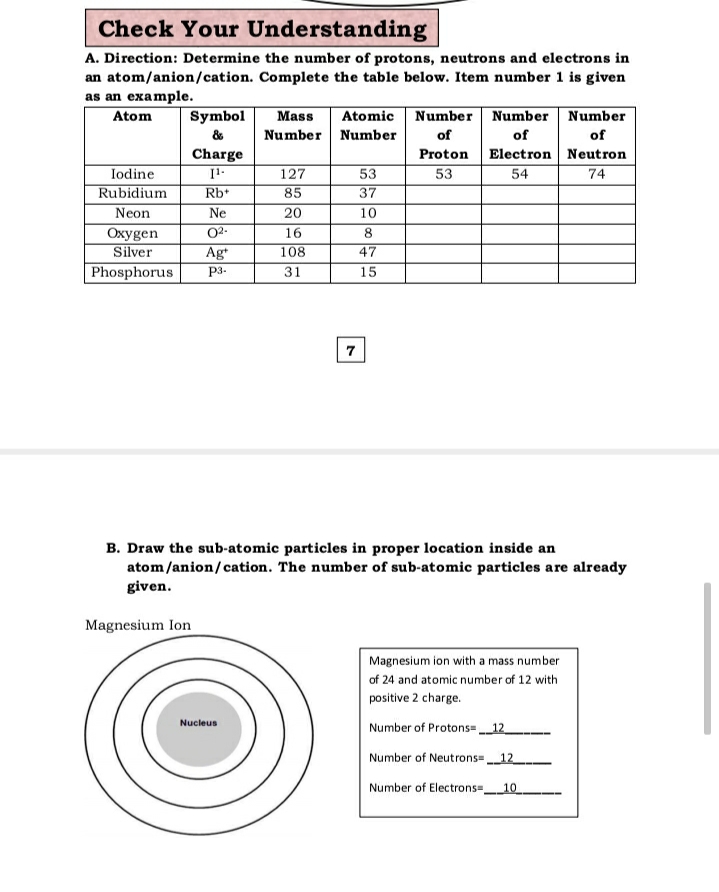
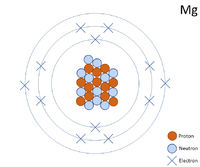
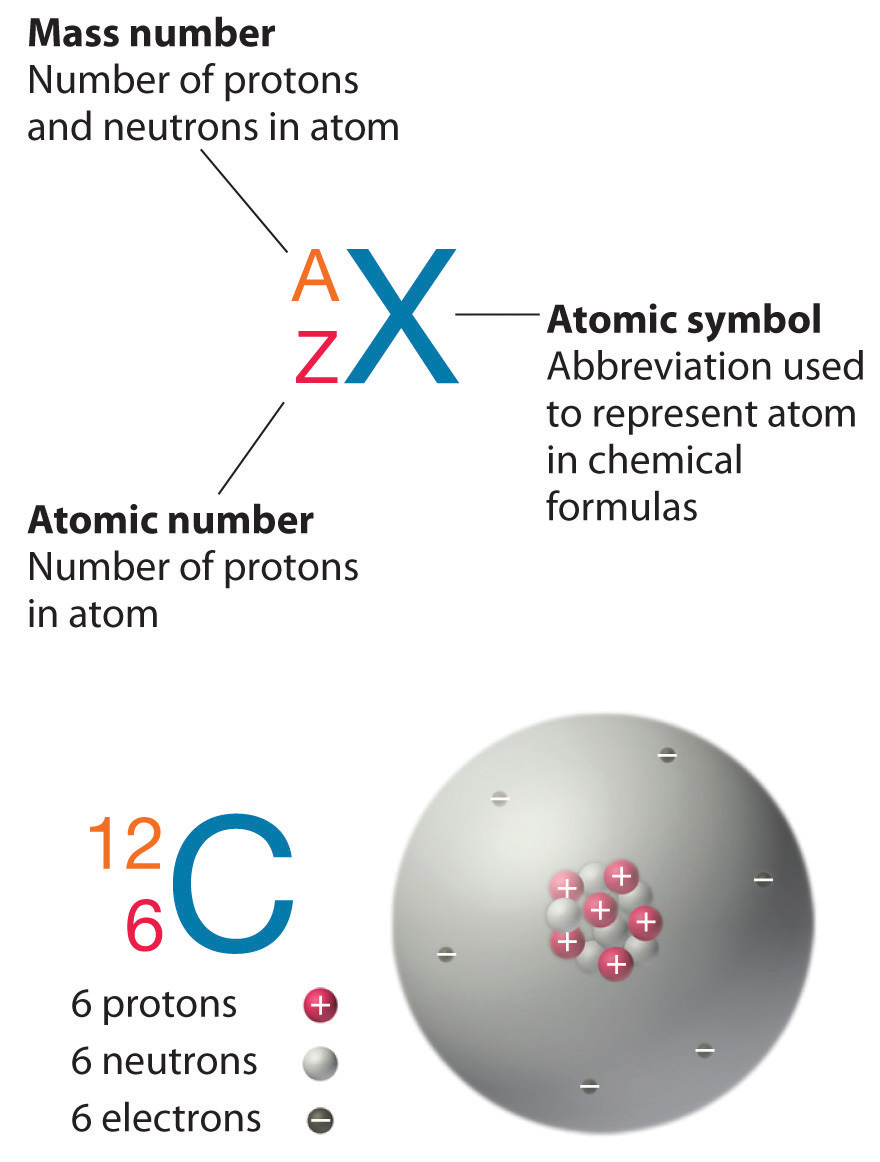show the electron distribution of a magnesium atom and a magnesium ion diagrammatically and also give - Brainly.in

What is the Difference Between Magnesium Atom and Magnesium Ion | Compare the Difference Between Similar Terms

The magnesium atom with its protons, neutrons, and electrons is displayed. | Download Scientific Diagram
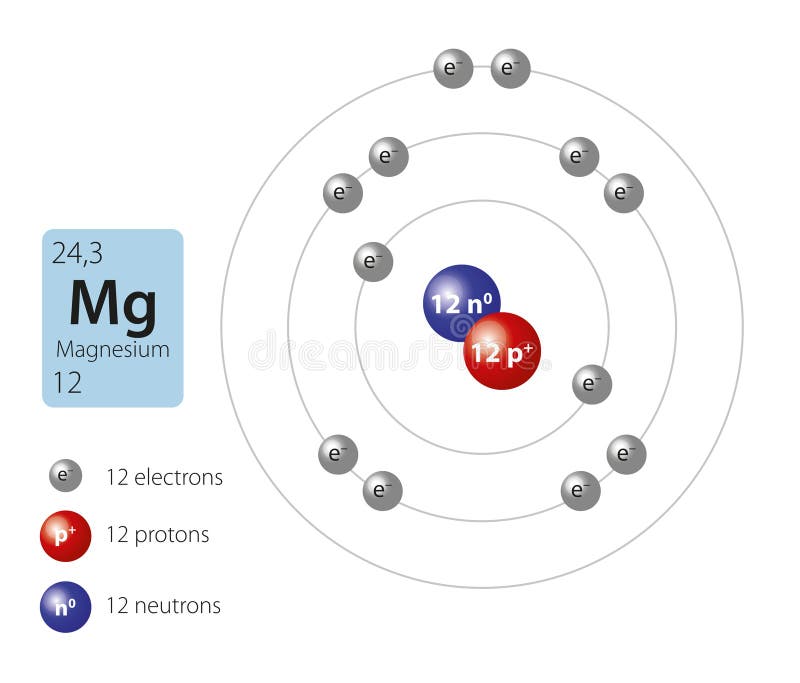
Magnesium Atom Stock Illustrations – 614 Magnesium Atom Stock Illustrations, Vectors & Clipart - Dreamstime

SOLVED: Complete the ionization reaction equation Pt - + 2e" 0 + =02- F+e - Mg Mg?+ + Determine the charge on each Ion strontium with 36 electrons platinum with 74 electrons

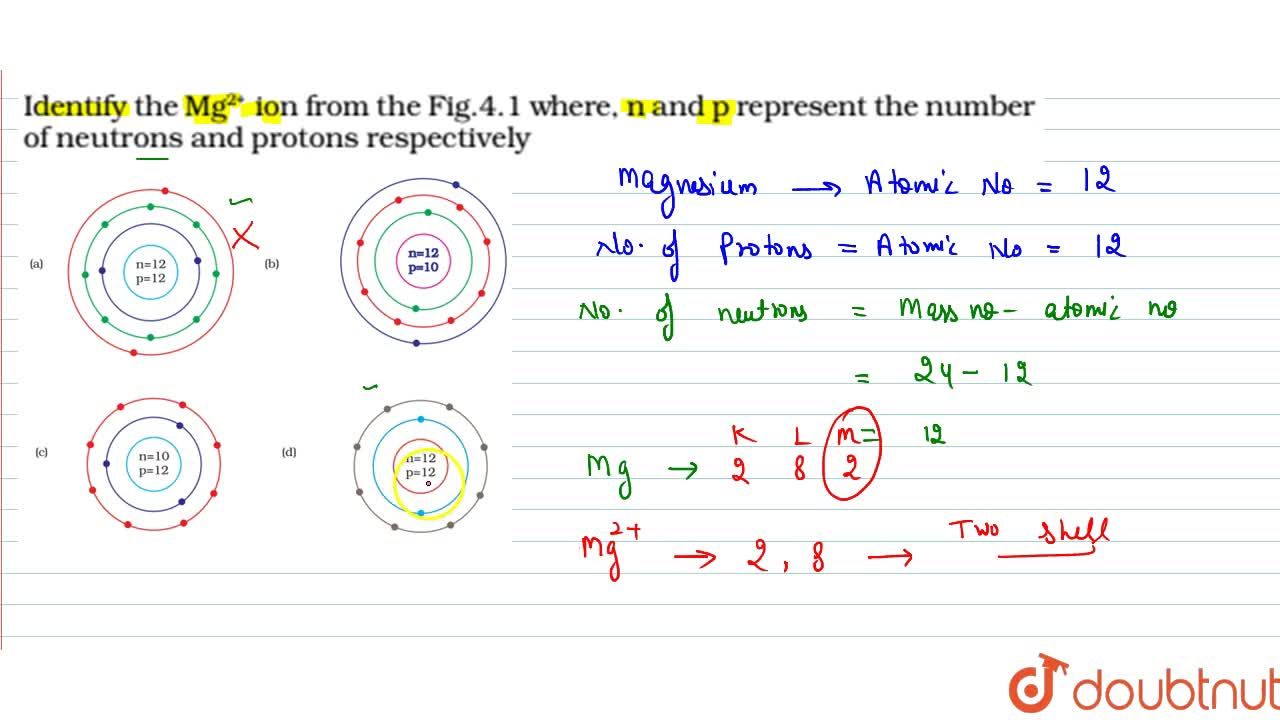
Identify the Mg2+ ion from Fig. where n and p represent the number of neutrons and protons respectively.
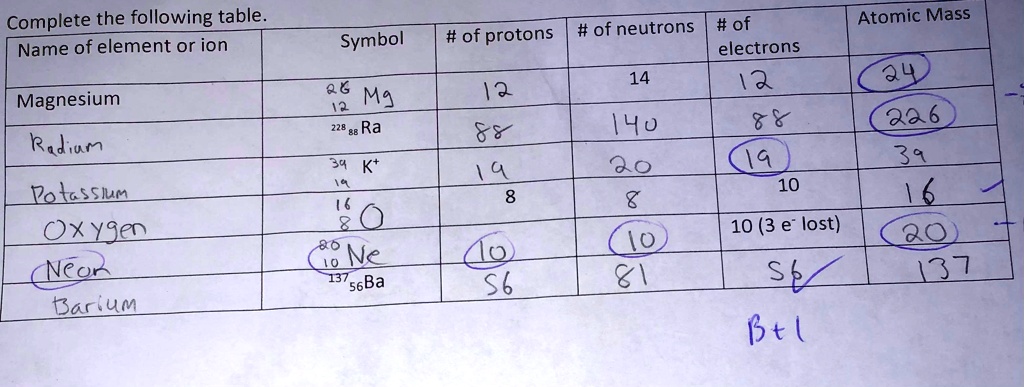
SOLVED: Complete the following table Name of element or ion #of neutrons #of #of protons electrons 14 12 12 140 8 Atomic Mass Symbol Magnesium Radiun 06 Mg ee Ra 226 3
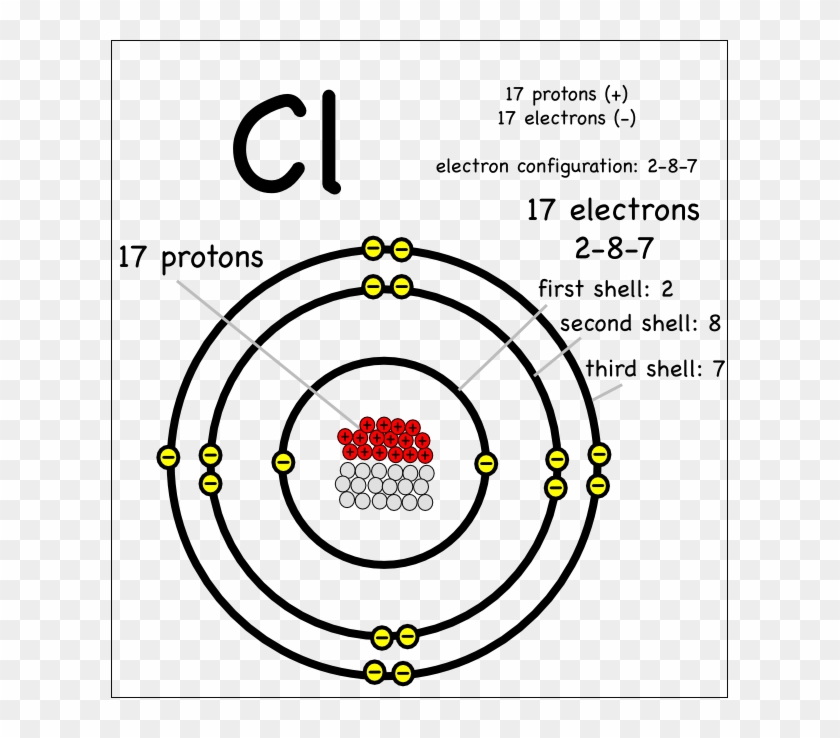
An Introduction To Ionic Bonding Montessori Muddle - Mg Element Protons Neutrons Electrons - Free Transparent PNG Clipart Images Download